Henderson man calls COVID treatment life-saving
Henderson resident Lynn Fetterly has a story he’s telling anyone who will listen.
It’s about how in the nick of time he learned of an experimental treatment for COVID-19 for some higher-risk patients that he believes saved his life and that of his wife.
On Jan. 5, Fetterly, who is 73, began to experience cold symptoms that within a few days had escalated to a bad sore throat, achy joints, a constant cough and difficulty breathing. A test on Jan. 7 confirmed that he had COVID-19.
“I could see my physical condition deteriorating” to where he didn’t have the energy to so much as talk on the phone, recalled Fetterly, who had a heart attack last year.
Around this time, he read a Review-Journal article about how the federal government in partnership with Sunrise Hospital and Medical Center had set up a temporary center in Las Vegas to provide monoclonal antibody treatment to patients in the early stages of a coronavirus infection.
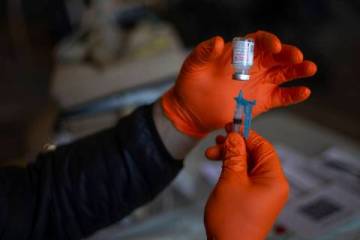
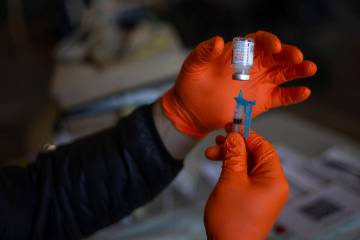
Fetterly qualified for the treatment, which consists of administering intravenously molecules produced in laboratories that act as substitute antibodies, mimicking the immune system’s response to infection.
The day after receiving the treatment on Jan. 10, Fetterly said, he began to feel significantly better. Three days later his symptoms were reduced to a cough and some heaviness in his chest. Within five days he felt “95 percent back to normal.”
“The infusion gave me artificial antibodies to immediately tackle it,” Fetterly said. “So yes, I’m absolutely convinced that that was the salvation. And I really don’t know if my own body would have fought it off naturally.”
When Fetterly’s wife, Melody, also became ill from the coronavirus, she, too, received the treatment and has recovered with minor lingering fatigue.
Standing up the center
Dr. Steven Merta, chief medical officer at Sunrise Hospital, said he “couldn’t be more elated” to hear the Fetterlys’ story of recovery.
It was the hope of keeping people out of the hospital — at a time of record volumes of COVID-19 hospitalizations — that motivated Merta and Sunrise staff to accept the call to create the center from the ground up in seven days. The effort was in partnership with the office of the assistant secretary for preparedness and response in the U.S. Department of Health and Human Services.
The center, only the third of its type in the federal program, opened on Jan. 8. Since, the federal team that helped open the center has handed off its management to the Sunrise staff.
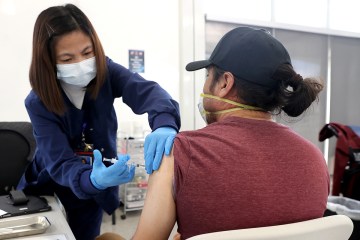
The center is in a big, white, climate-controlled tent outside the hospital. It can treat about 30 patients per day in shifts of 10 patients each. At an appointment, medicines are administered through an intravenous treatment called an infusion. The infusion takes about an hour, followed by an hour of observation. The one-time appointment takes about 2½ hours.
Monoclonal antibody treatments have been shown to decrease hospitalization rates in people at highest risk from severe disease from COVID-19 by 70 percent, according to information from the federal health agency.
At the Sunrise center, patients are treated with the Eli Lilly drug bamlanivimab, authorized Nov. 9 for emergency use by the U.S. Food and Drug Administration. It can be used to treat adult patients within 10 days of testing positive if they are at high risk for progressing to severe COVID-19 or hospitalization due to certain risk factors.
These risk factors include body mass index of 35 or greater; chronic kidney disease; diabetes; disease; receiving immunosuppressive treatment; 65 years of age or older; 55 years of age or older with cardiovascular disease, hypertension, COPD or another chronic respiratory disease.
The FDA also has authorized for emergency use Regeneron’s cocktail of two antibodies, casirivimab and imdevimab, which was used to treat former President Donald Trump.
‘Skeptic at heart’
The center had treated about 300 patients, none of whom to Merta’s knowledge had later required hospitalization at Sunrise. A federal team last week returned to the hospital to gather more data and to review operations to create a playbook for opening additional centers around the country.
Merta said that he is “definitely a believer” in the treatment from what he has seen and read, but is also a scientist and so a “skeptic at heart.”
“I too want that data that shows me that it is absolutely effective,” he said. “But based on what I’m seeing and hearing and watching, I do believe that the data will eventually show that this is absolutely essential and effective.”
Despite early promising results, adoption of the treatment across the country was slow last year, with some doctors saying they wanted to see more clinical data on the effectiveness. In late January, both Eli Lilly and Regeneron released further favorable study data, according to The Associated Press. The data had yet to be reviewed by the scientific community.
The slow adoption also has been attributed to physicians’ lack of familiarity with the new treatment as well as patients’ limited interest in being treated at the onset of the disease, when it is unclear how ill they might become from the virus. Some facilities also may not have the space and the staff to administer the treatments.
There are about 20 hospitals and other centers in Nevada authorized to provide the monoclonal antibody treatment, according to the National Infusion Association’s website.
The monoclonal antibody treatments are the only FDA-authorized medications that can be used outside a hospital to change the course of a COVID-19 infection, said David Wuest, executive secretary of the Nevada State Board of Pharmacy.
“These medications can make a difference in somebody’s life,” Wuest said in an email. “Patients that recently tested positive for COVID, that meet the criteria, should be evaluated by their health care provider to determine if the medications are a treatment option.”
After reading the Review-Journal story, Fetterly contacted his doctor, who referred him to the treatment program.
“I tell anybody who I meet or talk to that I’ve had COVID. I say, ‘I want to tell you what happened with me.’ I tell him the story.”
Referring medical providers may call Sunrise at 702-961-9075 to schedule a patient.
Contact Mary Hynes at mhynes@reviewjournal.com. Follow @MaryHynes1 on Twitter.