Las Vegas Valley kids, 12 to 17, sought for COVID-19 vaccine trial
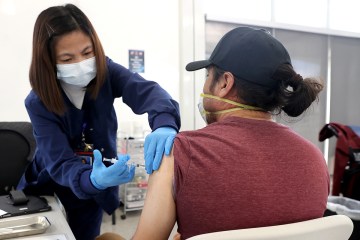
A Southern Nevada research center is looking for kids ages 12 to 17 to join a clinical trial testing the effects of a new COVID-19 vaccine on this age group.
The Wake Research-Clinical Research Center of Nevada is enrolling young participants for a trial involving the Novavax vaccine, which is expected to become the fourth vaccine authorized for emergency use in the U.S.
Despite evidence that the vaccine is effective and without major side effects, finding adolescents for the trial may be a challenge, the study’s principal investigator said.
Parents can be “reluctant to put their kids in a study where there’s blood draws and discomfort,’’ said investigator Dr. Michael Levin, a Henderson pediatrician. Not only the parents, but the children themselves must be willing.
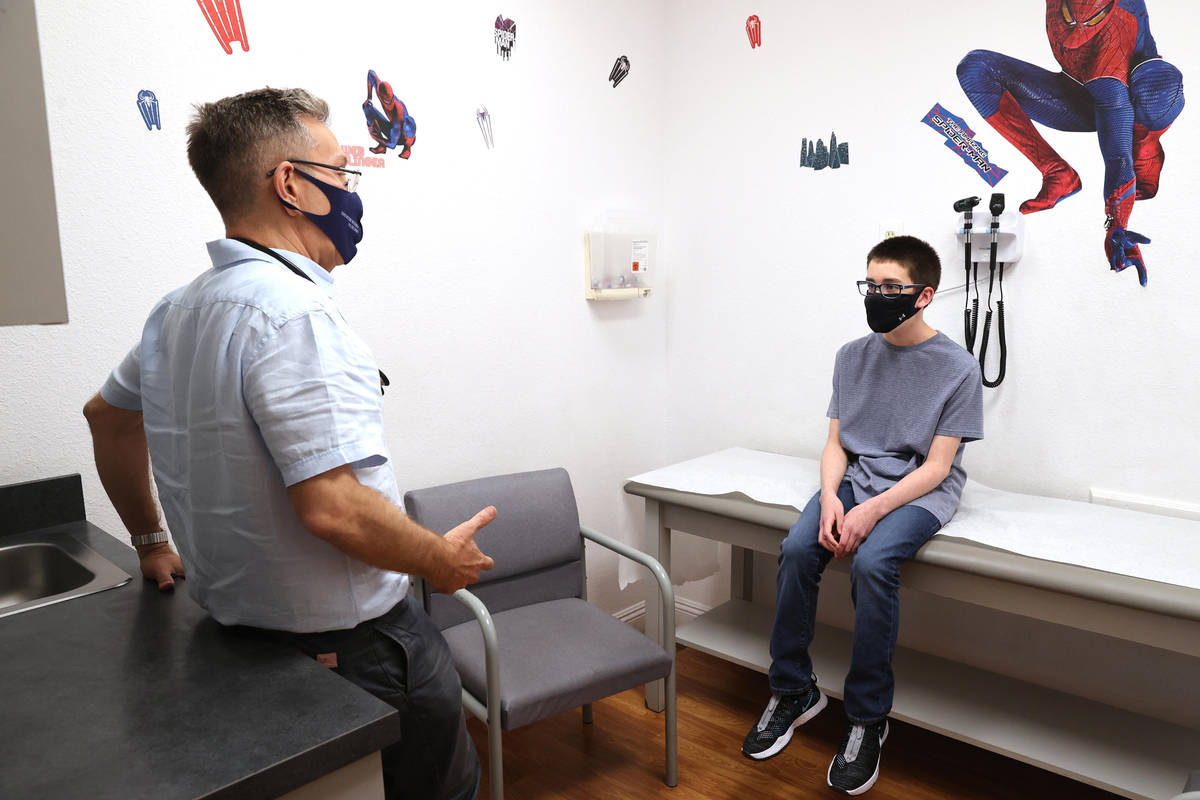
Sixth grader Trent Thurman said he didn’t have to be talked into joining the trial by his father, Wesley, who participated in a local trial of the Moderna COVID-19 vaccine.
“I want to get the vaccine,” said Trent, who is 12. Why? “So I can be safe and that other people can be safe.”
Does getting a shot bother him? “No, not really,” the Mannion Middle School student said.
Vaccine or placebo?
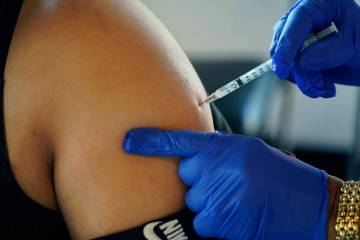
Participating in the trial involves getting two doses of either the actual vaccine or a salt-water placebo three weeks apart, as well as follow-up visits, blood draws and check-in phone calls.
Six months after getting the shots, those who were given the placebo will receive the actual vaccine in a “blinded crossover,” meaning participants won’t be informed whether they initially received the vaccine, Novavax said in a news release Monday about the study. Participants will be monitored for up to two years after their final dose.
The company is seeking as many as 3,000 adolescents to participate in the trial at 75 sites across the country, the news release said.
The local trial will be enrolling 60 to 100 participants, according to Levin.
Clinical trial participants receive monetary compensation, which typically amounts to $1,000 or less, said Levin, who did not provide an exact figure.
To be eligible, an adolescent needs to be in stable health, with any medical condition being well managed, he said. Those who have autoimmune diseases or compromised immune systems are not good candidates. Anyone who tested positive or has already been vaccinated for COVID-19 is ineligible.
Late-phase clinical trials in the U.K. showed the Novavax vaccine to be nearly 90 percent effective against the coronavirus overall and 86 percent against the U.K. strain or variant known as B.1.1.7, according to the Maryland-based biotech company. A midphase clinical trial in South Africa showed the vaccine to be 100 percent effective against serious disease but 49 percent effective against preventing disease from an immunity-evading variant, B.1.135, first spotted in that country.
A more traditional approach
The vaccine uses a more traditional technology than other vaccines authorized in the U.S., Levin said
The vaccine contains harmless pieces of the coronavirus’ spike protein. In response to these pieces, the body starts making antibodies that can neutralize the actual virus if a person becomes infected.
No serious side effects from the vaccine have been observed in the adult trials, Levin said. Minor side effects have included typical vaccine reactions such as soreness at the injection site and fatigue.
However, there is always the potential for a rare but serious side effect. Use of the Johnson & Johnson COVID-19 vaccine was temporarily halted after extremely rare but potentially life-threatening blood clots in the brain were diagnosed in several patients, including an 18-year-old Henderson woman, who had received the shot.
Although children are far less likely than adults to become seriously ill from COVID-19, Wesley Thurman said he’s more concerned about potential ill effects his son could suffer from the disease than possible side effects.
He is also a believer in vaccine trials as a way to help the community move forward.
“It’s part of helping the whole city get back to business, helping schools get back to a normal flow where, hopefully, kids will be able to start next school year full time, in person and, hopefully, without mask requirements, as well,” he said.
Only the Pfizer-BioNTech vaccine is currently authorized in the U.S. for anyone under age 18. It is available to people 16 and older. The Food and Drug Administration is preparing to authorize its use for 12- to 15-year-olds by early next week, The New York Times reported Monday.
For more information on the local Novavax clinical trial, call 702-893-8968.
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.