Drugmaker, U.S. regulators reach deal for dying 7-year-old to receive trial drug
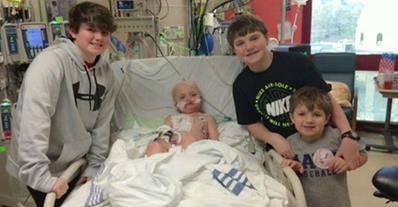
A dying 7-year-old boy who is suffering from a viral infection could receive an unapproved medical treatment as early as Wednesday after a drugmaker struck a deal with U.S. regulators to provide the medicine.
Pharmaceutical company Chimerix Inc, lobbied by supporters of Josh Hardy in a campaign that has gained national attention, will make him the first patient in a 20-patient pilot trial of open-label brincidofovir (CMX001).
The drug, used to treat adenovirus infections in patients with weakened immune systems, has not been approved by the U.S. Food and Drug Administration and was initially withheld by the Durham, N.C.-based company.
“Being unable to fulfill requests for compassionate use is excruciating, and not a decision any one of us ever wants to have to make,” Chimerix President and Chief Executive Kenneth Moch said in a statement on Tuesday.
“It is essential that each individual in a health crisis be treated with equal gravity and value.”
Hardy remains in a critical condition in intensive care at St. Jude Children’s Research Hospital in Memphis, Tenn., after a bone marrow transplant left him with the adenovirus infection.
“Glory to GOD! They are releasing the drug for Josh!” his mother, Aimee Hardy, wrote in a Facebook post after the announcement.
A post on the “Savejosh” Facebook page thanked those who had helped get the word out about Josh and the drug.
“Thank you to every member of Josh’s Army,” the post said. “The world has heard you and because of you Josh and many others will have the opportunity to receive CMX001 (Brincidofovir) the life saving antiviral drug made by Chimerix.”
The “Savejosh” Facebook page had garnered more than 24,000 “likes” as of Wednesday afternoon. It was used to convey messages of solidarity with the ailing boy and his parents and to organize a rally at Chimerix headquarters.
Aimee is grateful for all the support her son is receiving, but told Fox News that Josh is running out of time.
“He’s still kind of weak, but I really am optimistic about the potential of this drug and I really feel like within a couple weeks we’re going to see a turnaround and get him in the right direction,” Aimee told Fox News. “It was a tremendous gift.”
The FDA-Chimerix agreement stemmed from a “compassionate use” policy clause that at times allows drugmakers to administer unapproved treatments to patients with life-threatening conditions.
CNN reported St. Jude said it was to receive the drug within 48 hours, but warned its safety and effectiveness has not yet been established for use in treating children.
“It is also important to understand that this remains a critical and complex medical situation,” the hospital said in a statement to CNN. “St. Jude will continue to pursue state-of-the-art treatment for Josh and all of our patients. We are grateful for the efforts of Chimerix, the FDA and many others who worked to achieve this outcome. We ask that you continue to keep Josh and his family in your thoughts.”
At just 9 months old, Josh was diagnosed with a rare cancer that affects only 15 kids a year, the San Francisco Chronicle reported.
Josh has beat cancer in his thymus, lung and bone marrow, according to CNN.
Last year, Josh underwent a bone marrow transplant that led to more complications and was admitted to the ICU in January for heart failure, according to the San Francisco Chronicle.
Kira Terry contributed to this report.