Can an experimental drug prevent dementia from Alzheimer’s?
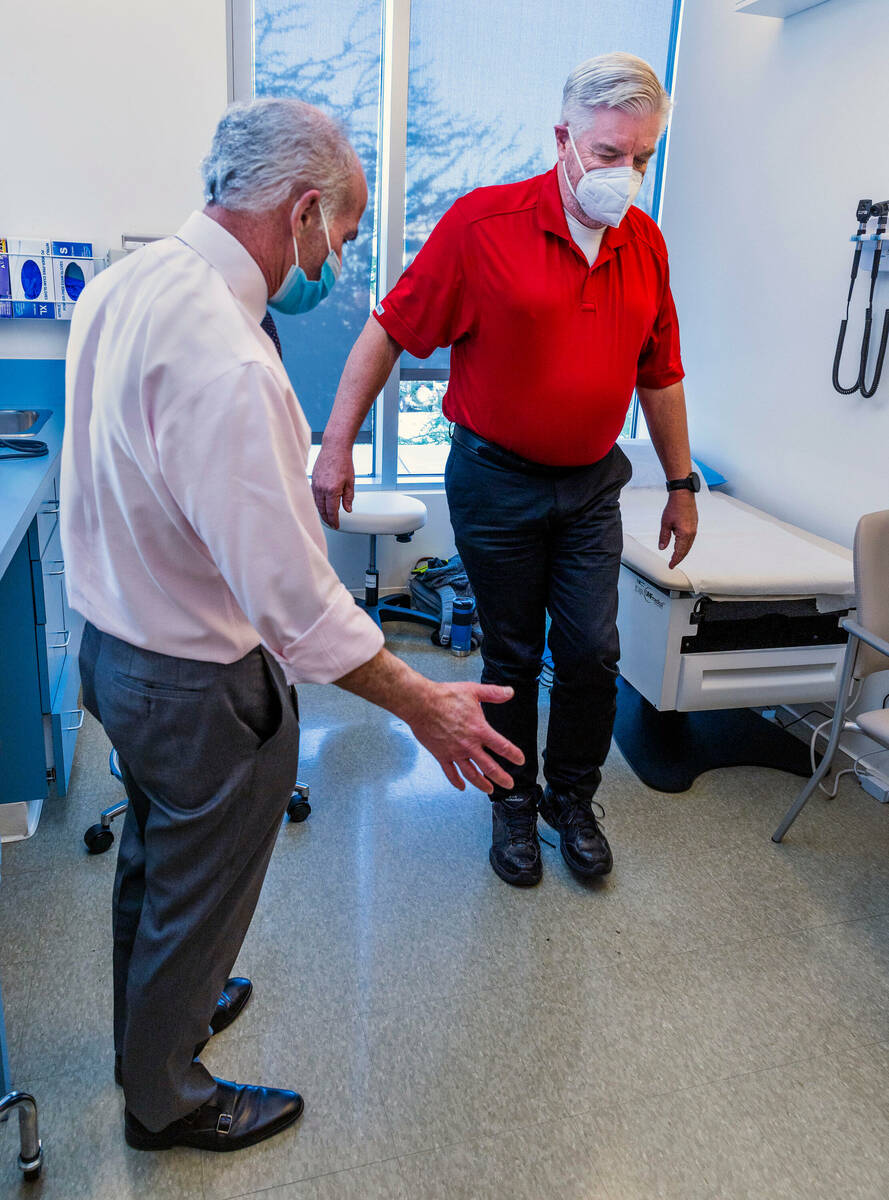
Bob Lathrop’s wife likes to rib him when he can’t find his keys or misplaces a credit card.
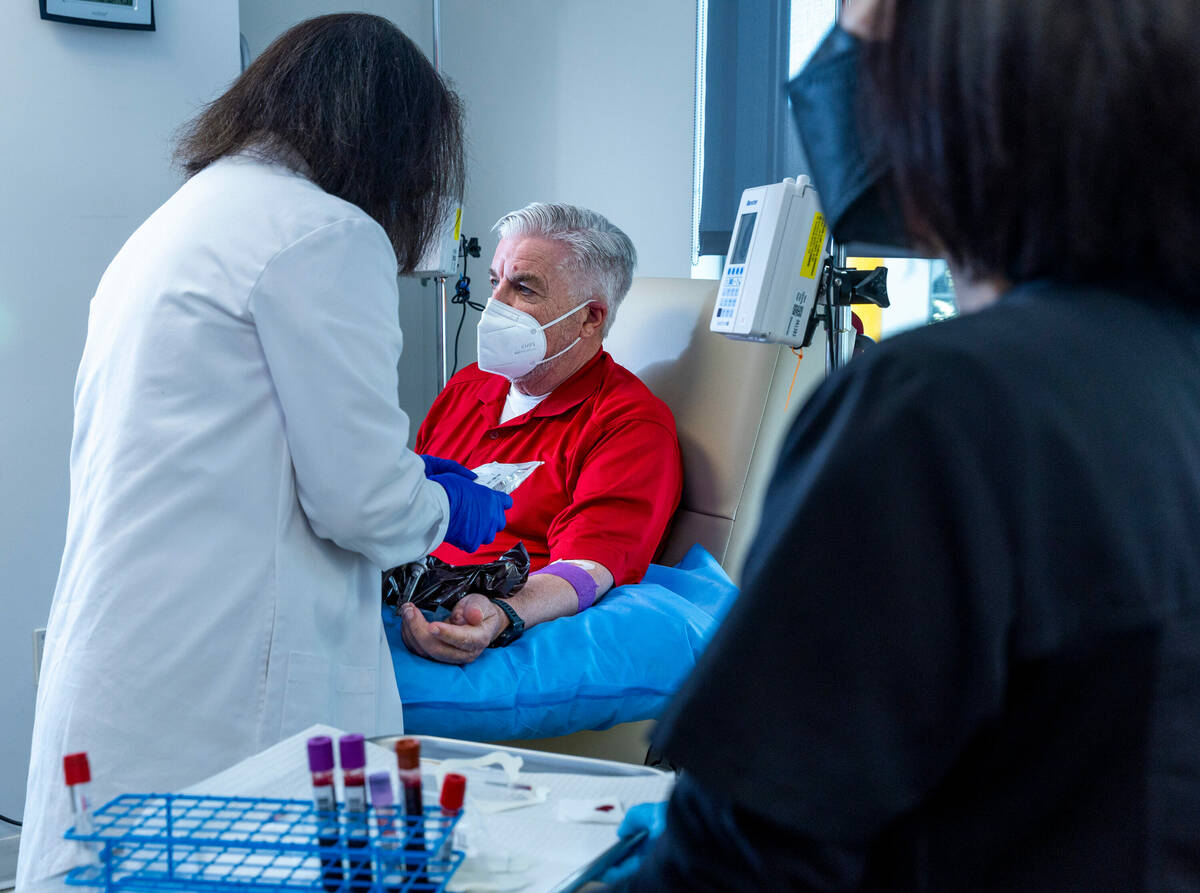
She’ll tell her husband, a participant in a clinical trial in Las Vegas for an experimental Alzheimer’s drug, that he must be getting the placebo and not the actual drug.
Lathrop, 68, isn’t troubled by his occasional forgetfulness. And he says he isn’t overly concerned about the possibility of developing Alzheimer’s. This despite a family history of dementia, along with scans showing that his brain has accumulated beta-amyloid plaque, a hallmark of Alzheimer’s. Not everyone with the plaque will develop the disease.
He won’t fret over what he can’t control. “Whether I get Alzheimer’s or not, it’s gonna happen,” said the Las Vegan, who sells security systems to businesses. “It’s a fate thing. It’s a family thing. It is what it is.”
The AHEAD clinical trial at the Cleveland Clinic Lou Ruvo Center for Brain Health is for people like Lathrop, who have a build-up of plaque but normal cognitive function. The study is testing whether the experimental drug lecanemab can prevent the devastating loss of memory and mental function associated with Alzheimer’s.
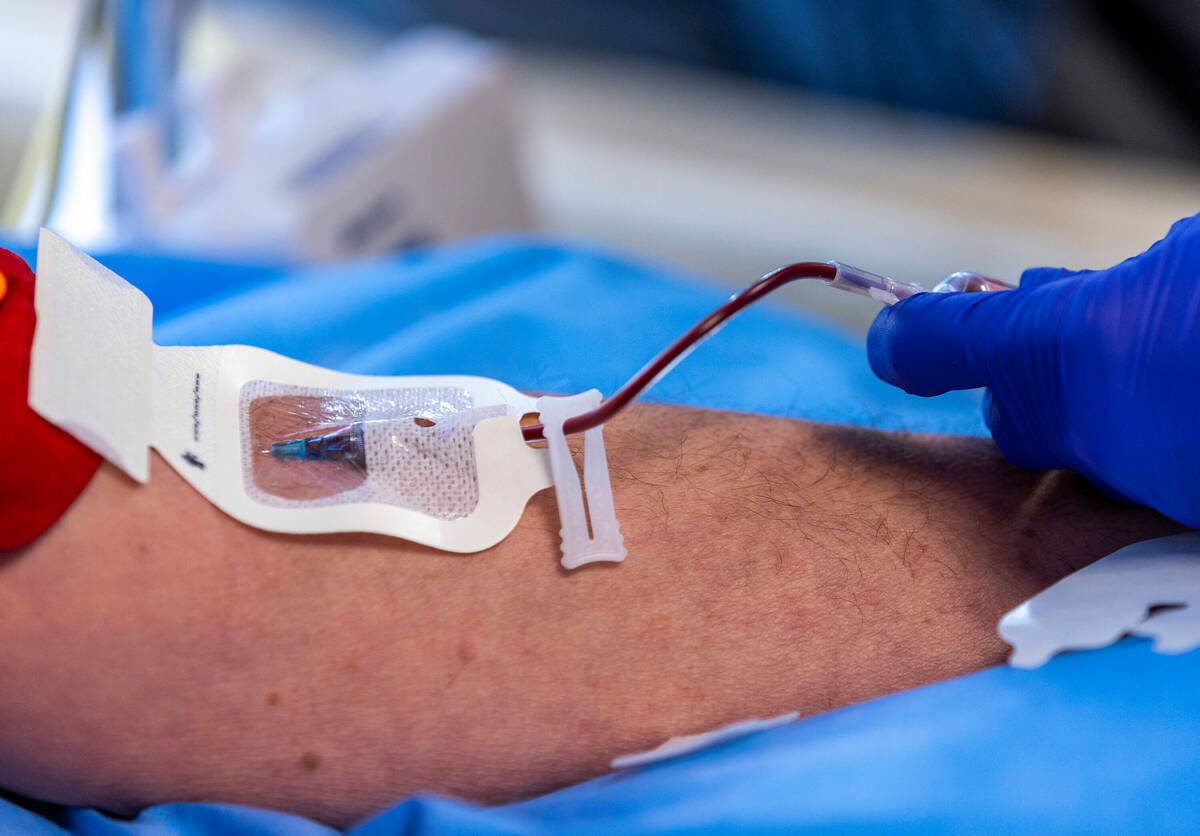
Lecanemab, developed by Japanese drugmaker Eisai and U.S. partner Biogen, contains monoclonal antibodies designed to remove beta-amyloid from the brain and in doing so, slow progression of the disease.
Data released last month from a different study, the CLARITY clinical trial, demonstrated that the drug removed beta-amyloid and slowed cognitive decline in patients with mild cognitive impairment from Alzheimer’s.
The data showed that lecanemab delayed patients’ worsening by about five months over the course of the 18-month study, Eisai’s Dr. Michael Irizarry told The Associated Press. Also, lecanemab recipients were 31 percent less likely to advance to the next stage of the disease during the study.
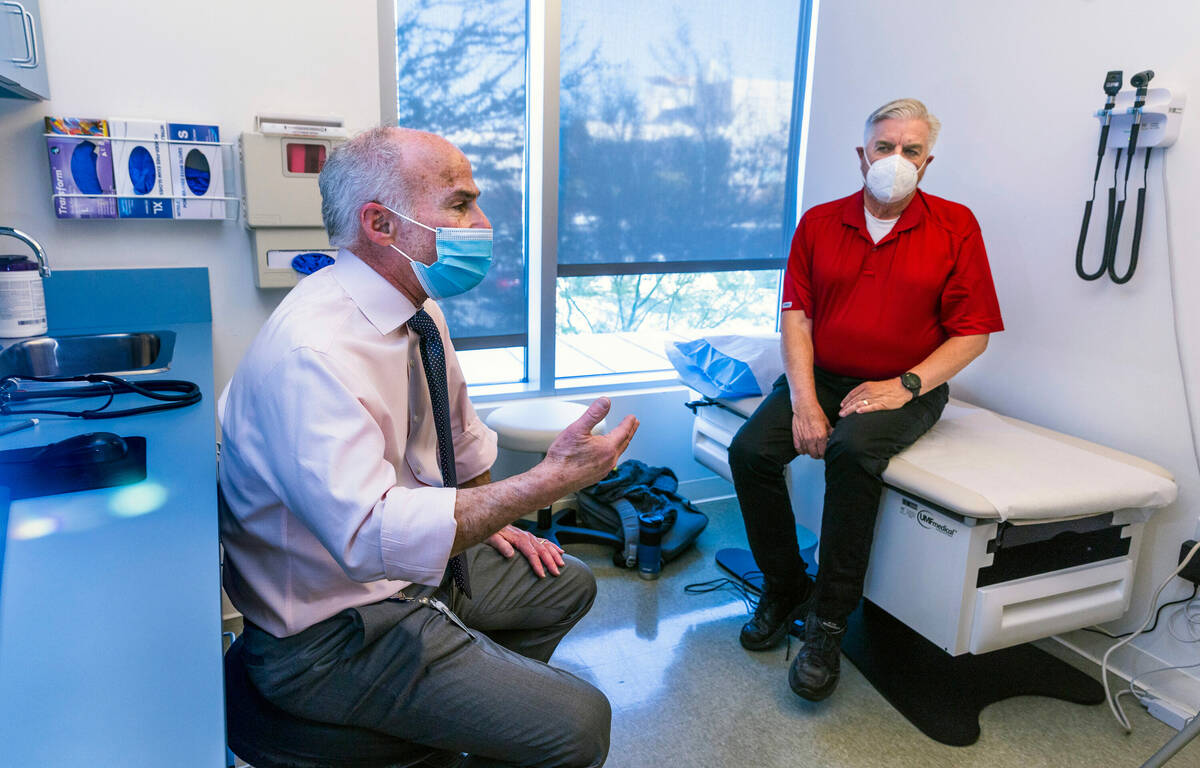
The CLARITY study data indicated that the progression of the disease was reduced by 25 percent over the 18 months, said Dr. Charles Bernick, the Ruvo Center’s lead investigator for the AHEAD study. That benefit, he said, could compound over time.
Early intervention
The AHEAD trial at the Ruvo Center is looking at whether effective intervention can occur before patients have symptoms.
A major advancement has been developing the tools “to identify people who are starting to have Alzheimer’s disease years before they even have symptoms,” Bernick said. “And then the thought is, OK, so maybe we can intervene earlier. Maybe removing amyloid when it first starts accumulating, before people have any symptoms, may significantly change the trajectory of the disease. … What you’re trying to prevent is the progression to dementia, to the stage of the disease where you just can’t function independently.”
The Ruvo Center is seeking more participants for the five-year trial. Those eligible for consideration include healthy, nonsmoking adults ages 55 to 88 who have not been diagnosed with Alzheimer’s.
An example of a good candidate is someone age 65 with a parent diagnosed with Alzheimer’s, who may be beginning to have more trouble remembering things, Bernick said. Since the disease has a genetic component, such a person might have more motivation to participate in the study.
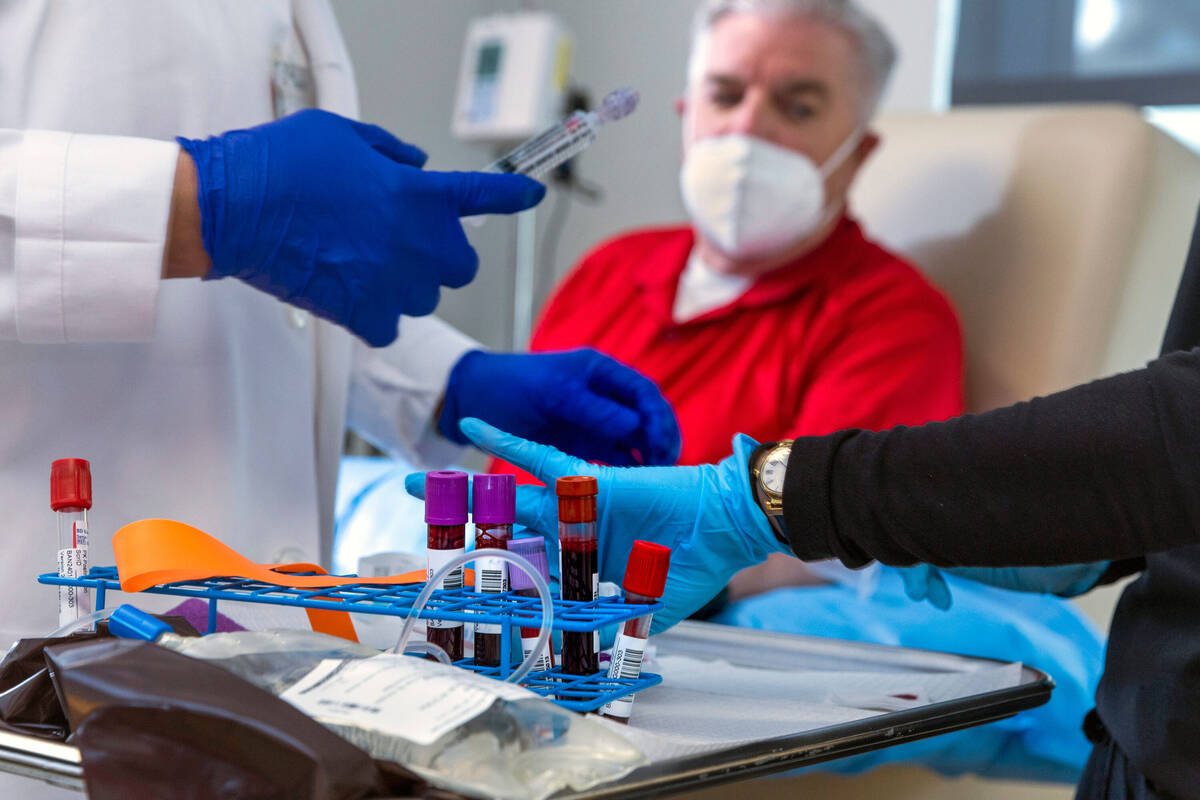
Potential candidates are screened for the amyloid plaque, first through a blood test and, if that’s positive, by imaging of the brain.
If individuals are negative for the plaque, disqualifying them from the study, “at least you know that you do not have any amyloid accumulated in your brain, thus your risk of Alzheimer’s’s disease, at least for the next 10 years, is probably pretty low,” Bernick said.
Those selected for the trial could receive a cutting-edge drug years before it could potentially become available to members of the public without symptoms of the disease. The FDA is expected next year to consider approving its use in Alzheimer’s patients with mild cognitive impairment.
First drug approved
The FDA so far has approved only one amyloid antibody: Biogen’s aducanumab, also known by the brand name Aduhelm. The federal agency gave its conditional approval for the drug last year in the face of conflicting evidence about its effectiveness and against the advice of its advisory committee. It was the first drug to be approved designed to actually slow progression of the disease and not simply treat symptoms.
Doctors have not widely prescribed Aduhelm in part because of its high cost. Medicare decided it would cover Aduhelm only for patients enrolled in approved clinical trials.
Some of the controversy over Aduhelm stemmed from the manner in which the studies were done, Bernick said. In contrast, the rigor of the CLARITY study of lecanemab was such that he’s heard no criticism of it.
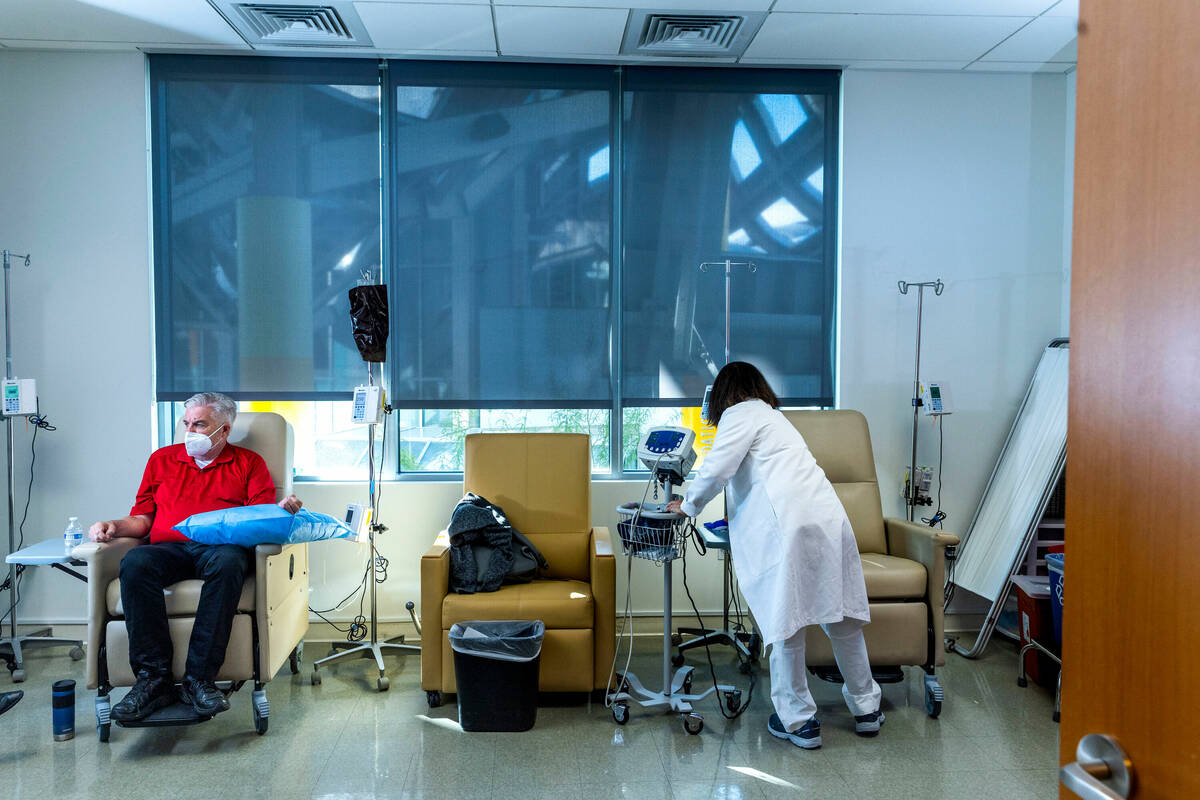
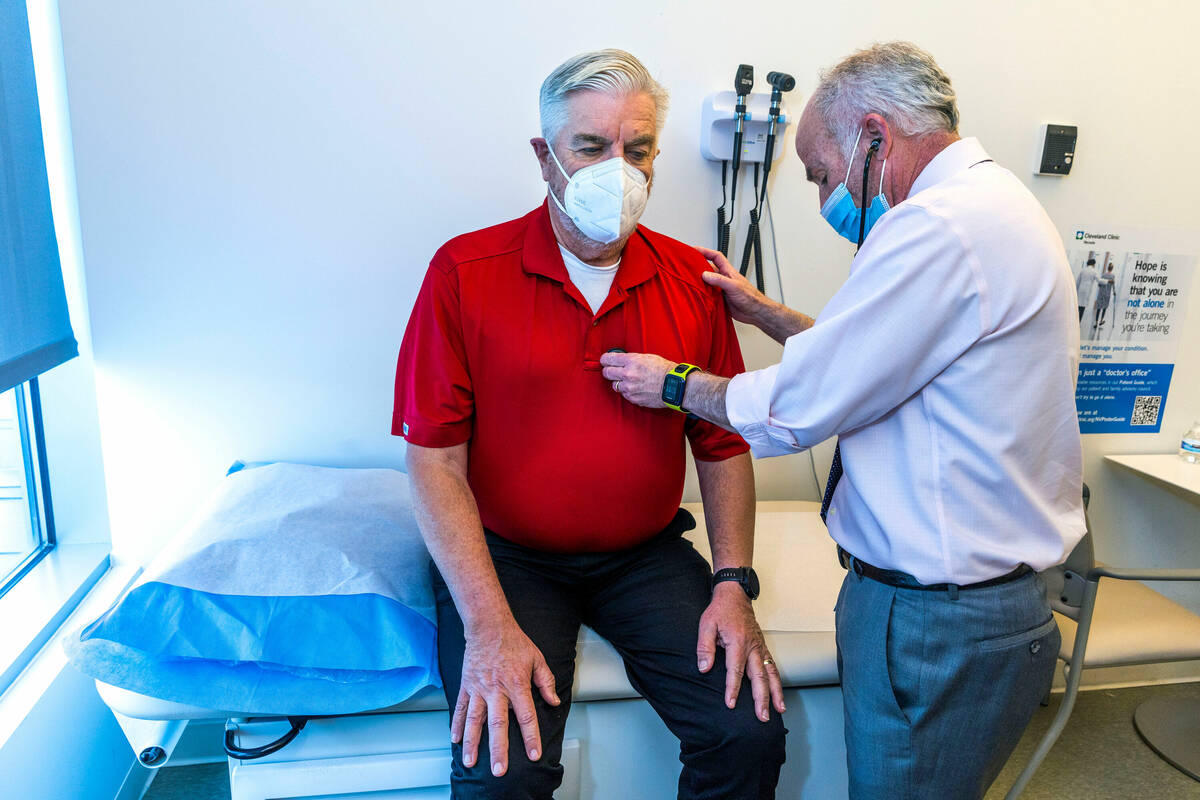
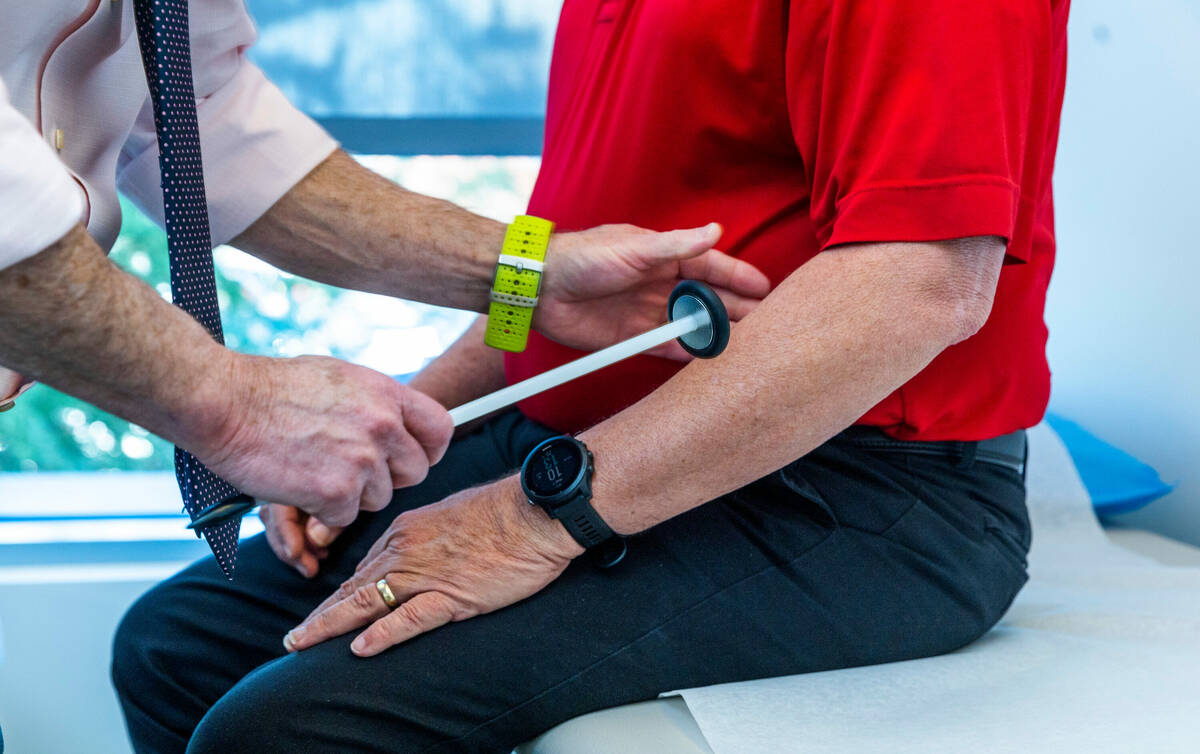
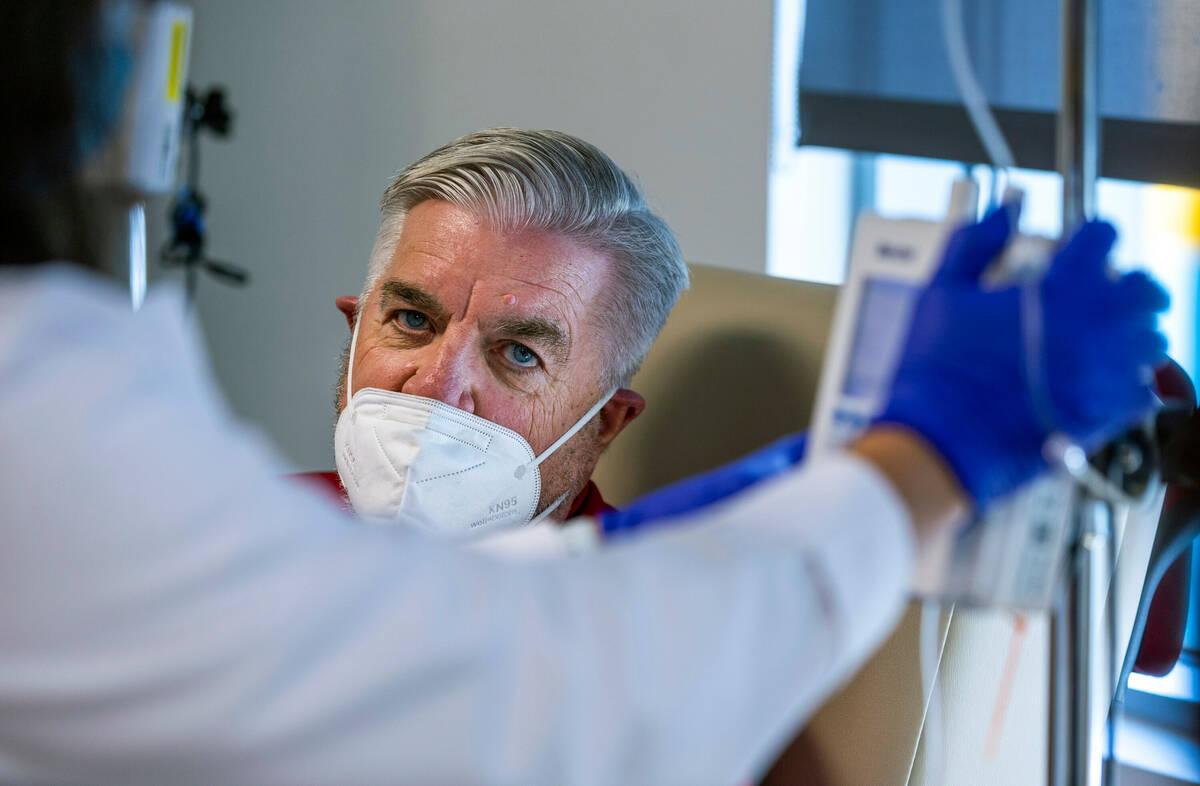
Participants in the AHEAD trial will receive an infusion of either the drug or saline every month or every two weeks, depending on the amount of plaque in the person’s brain. Participants are checked for potential side effects, such as brain swelling. Their cognitive and functional ability is measured and tracked.
Lathrop, a participant in a previous Ruvo brain study on chronic traumatic encephalopathy, said he doesn’t care whether he gets the actual drug during the clinical trial or the placebo. What he cares about is making a difference.
He has gone through the heartache of seeing both his mother and grandmother develop dementia.
“I want to try to make sure people down the road don’t have to go through this experience,” he said.
“This may not be the answer,” he said about the drug. “This may not be the one thing that solves everybody’s problem. But it’s going to be a step forward.”
Those interested in being considered for the AHEAD clinical trial can visit www.healthybrains.org/AHEAD or call 702-701-7944.
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.