Las Vegas center is site for COVID-19 vaccine testing
A return to normal could depend on the quiet work underway in places like the small, gray stucco building east of the Strip.
Tucked in the back of a Sahara Avenue commercial center, near a storefront church, a day spa and a homeless person’s lean-to, the Wake Research-Clinical Research Center of Nevada is among the sites conducting final-phase trials for a vaccine to prevent COVID-19.
The Las Vegas location is one of nearly 100 sites across the country engaged in a trial for a vaccine developed by Cambridge, Massachusetts-based biotech company Moderna in conjunction with the National Institute of Allergy and Infectious Diseases, the federal agency led by Dr. Anthony Fauci that is part of the National Institutes of Health.
This vaccine trial is the first to be implemented under Operation Warp Speed, an initiative led by the U.S. Department of Health and Human Services to accelerate the manufacture and distribution of COVID-19 vaccines and treatments. Where it typically has taken a decade to develop an effective vaccine, the goal here is to make one available by the end of the year.
“It’s clinical research on steroids,” said Dr. Michael Levin, a Henderson pediatrician with two decades of clinical research experience who is serving as the primary investigator at the Las Vegas site.
Vaccine or saline
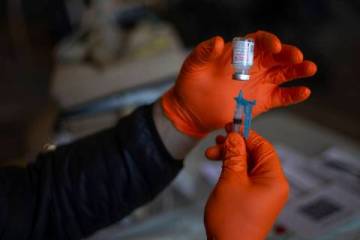
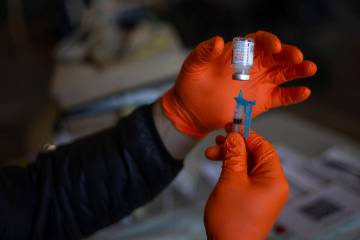
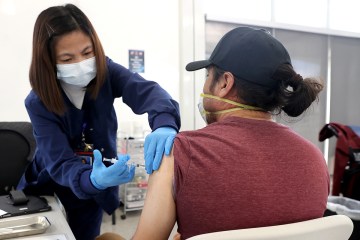
The center, which also has been tapped to conduct trials for other drugs to prevent and treat the disease caused by the new coronavirus, is recruiting as many as 500 Southern Nevadans to be among 30,000 participants across the country to receive either the trial vaccine or a placebo. Participants will receive two injections — both of either the trial vaccine or saline — 28 days apart. Neither the participants nor the trial investigators know who receives the actual vaccine.
Participants will be asked to provide nasal swab and blood samples during an initial screening and additional blood samples after each injection and at various times over the next two years, according to the National Institutes of Health. Scientists will use the blood samples to quantify the immune response.
Investigators will call participants after each injection to discuss any symptoms. They’ll provide participants with a diary to record symptoms and a thermometer for temperature readings.
Participants suspected of having COVID-19 will be asked to provide a nasal swab for testing within 72 hours. If the test is positive, they will be followed closely and referred for medical care if symptoms worsen, NIH said in a news release.
Earlier trial phases already have determined that the vaccine is safe and that it triggers an immune response, according to NIH.
“The question is, is that immune response going to be enough to protect somebody in the wild” outside of a laboratory setting? Levin said. “Do they get an infection, or do they get a milder case?”
The center is seeking a cross-section of individuals, with at least a quarter at higher risk for COVID-19 because they are 65 or older or have underlying health conditions, Levin said. However, participants cannot be acutely ill. The center is seeking not only retirees but essential workers who may be more likely to come in contact with people with COVID-19.
Ultimately, the immune response in participants receiving the trial vaccine will be compared to that of participants receiving the placebo.
So-called hot zones for COVID-19, where there’s a greater likelihood of infection, are being given priority for the trials, according to NIH. All six of the Wake Research sites across the country testing the Moderna vaccine were identified as coronavirus red zones, areas experiencing higher rates of disease transmission identified in internal documents prepared for the White House coronavirus task force.
Of mice and men
The Las Vegas center normally is involved in testing a wide range of vaccines, drugs and treatments. But now, much of the center’s work focuses on COVID-19. The center is preparing to conduct three additional COVID-19 vaccine trials, Levin said.
The center also is poised to conduct a trial on a treatment for reducing inflammation in patients with mild to moderate cases of COVID-19 who do not require hospitalization. The treatment is a supplement put in liquid aimed at preventing a “cytokine storm,” an inflammatory response by the immune system so severe that it can cause harm and even death.
The center also has been involved in studying COVID-19 testing devices, including a rapid test similar to a home pregnancy test.
It also will conduct two separate trials of a single treatment to prevent COVID-19: one study in people living with someone who has been diagnosed with the disease, and another in first responders who because of their work may be at greater risk of coming in contact with people who are ill with the virus.
The treatment involves genetically engineered antibodies produced in mice from human proteins. The antibodies can be injected like insulin.
“It’s not a mouse gene. It’s what a human would make against COVID,” Levin said.
Plasma with the antibodies of patients who have recovered from the coronavirus is being used to treat current patients but is in limited supply.
All these approaches may show promise, but Levin said his greatest hope is for a vaccine.
“The best thing would be a vaccine that works on everybody,” he said.
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.
How can I volunteer?
Those interested in participating in a Wake Research-Clinical Research Center study related to COVID-19 can text "COVID" to 702-357-9650 or visit covidstudies.org.