Las Vegas clinic offers outpatient COVID treatment as part of trial
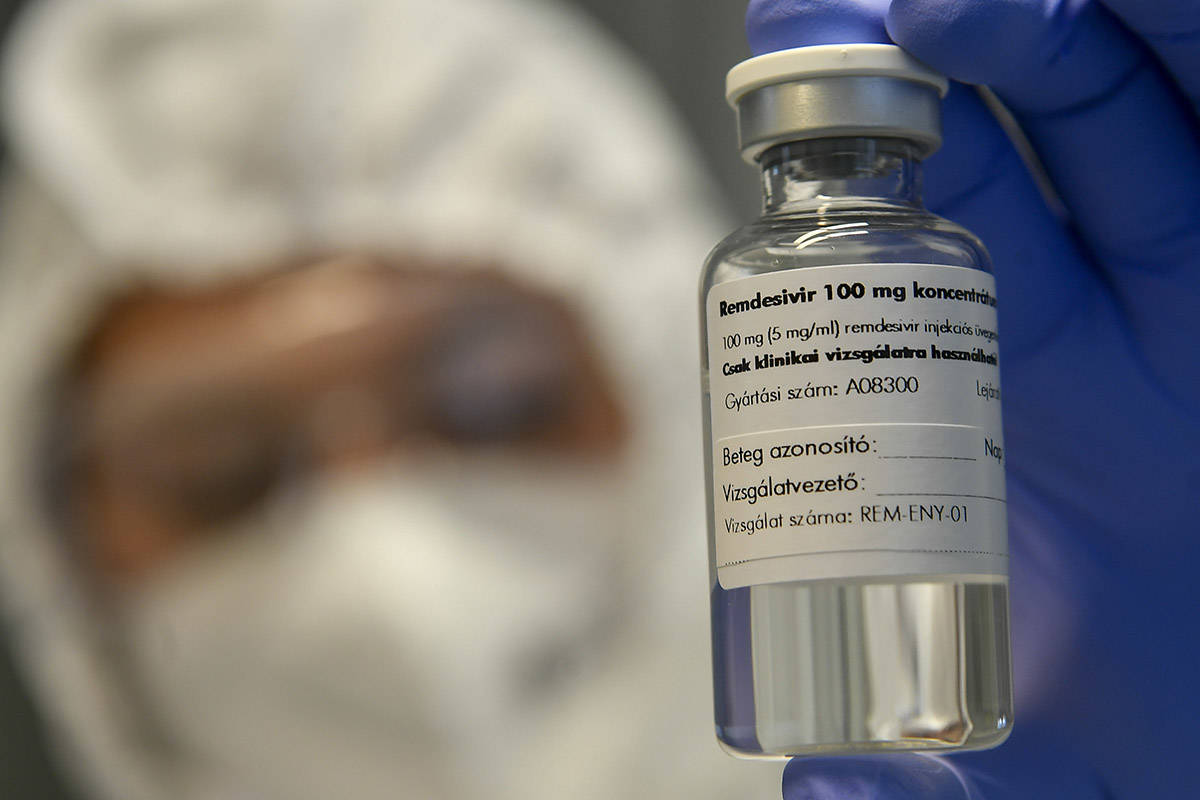
High-risk COVID-19 patients may be able to receive early remdesivir treatment at a Las Vegas clinic, one of 65 in the world offering the recently approved drug in an outpatient setting as part of an ongoing trial.
Dr. Atoya Adams, principal investigator at AB Clinical Trials, said receiving the drug early in an outpatient setting will ideally help keep patients out of hospitals and off ventilators.
The Food and Drug Administration in October approved the antiviral drug for emergency use on hospitalized COVID-19 patients. The trial offers qualified patients early access to the drug, but they also could receive a placebo. Patients as young as 12 can participate in the trial with a parent or guardian’s consent.
“The goal with this outpatient trial is to get high-risk patients in the first four days after their positive test,” Adams said. “We want to catch it in the beginning, rather than waiting for them to be hospitalized with more severe symptoms.”
To qualify for the trial, patients must test positive for COVID-19, experience mild to moderate symptoms and have a qualifying comorbidity, including hypertension, diabetes, heart disease, a history of strokes or obesity. Patients older than 60 or who are immunocompromised could also qualify, Adams said.
Bloodwork is also required, but if the patient hasn’t had it done within 90 days of the trial, a home health nurse can perform it so patients can stay quarantined, Adams said.
Las Vegas, which started the trial in late November, is one of 59 participating locations in the U.S. Two trials are underway in Denmark, three in Spain and one in the U.K. as well, according to clinicaltrials.gov.
The IV treatment is given in three, 90-minute sessions over the course of three days, Adams explained. Then patients will undergo a follow-up appointment at the facility a week later and a phone appointment the week after that.
Adams emphasized that there are no guarantees for those who receive the drug rather than the placebo, as it is still in the trial phase.
“It’s important for people to understand that we know they want answers, but we doctors just don’t have all the answers yet,” she said, adding that it takes time to fully approve a new treatment. “Acknowledging the differences between doctors’ hypotheses and factual knowledge is really important right now.”
She said it’s important that these new drugs undergo extensive trials, so doctors can be fully confident that they work before administering them to larger numbers of patients. Because of the ever-changing nature of COVID-19, she said, she can’t estimate how long the trial will last or whether the outpatient treatment will receive full FDA approval.
Any qualifying patients interested in participating in the trial can contact AB Clinical Trials at 702-233-4700 or abct@abclinicaltrials.com.
Contact Alexis Ford at aford@reviewjournal.com or 702-383-0335. Follow @alexisdford on Twitter.