Las Vegas hospitals drop hydroxychloroquine as COVID-19 treatment
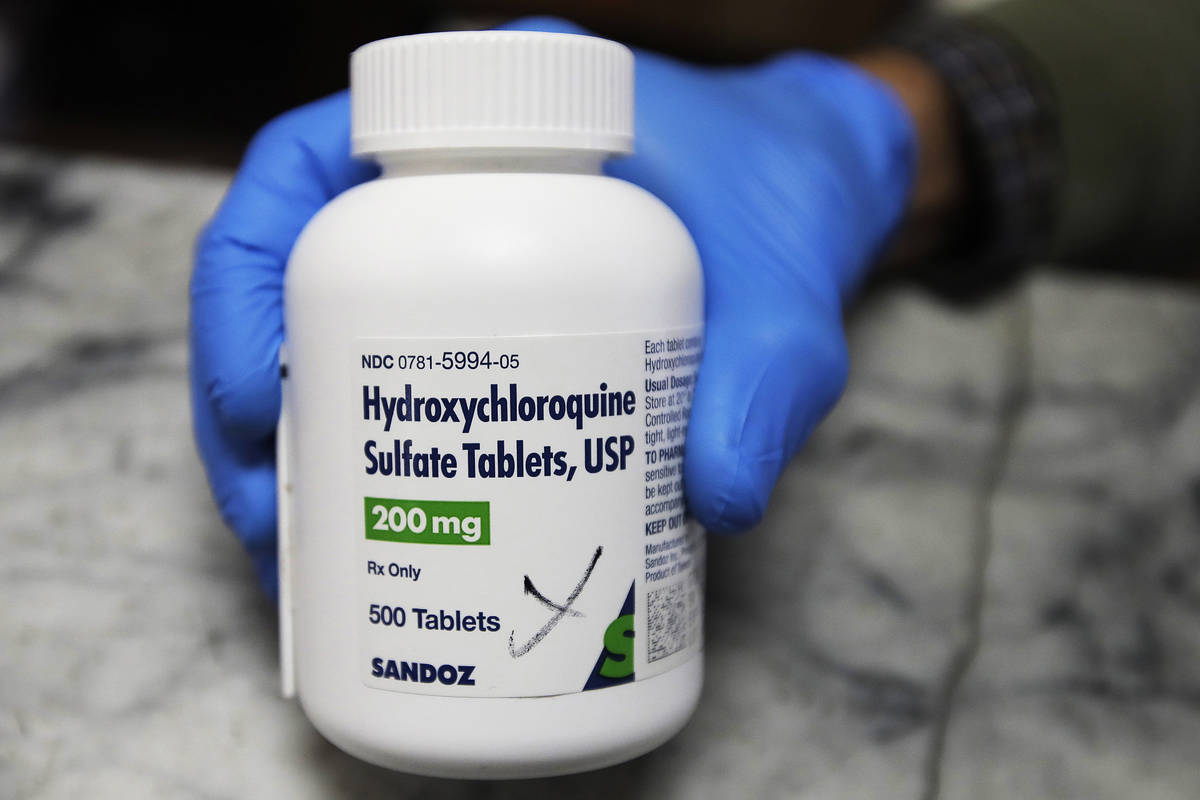
Despite early promise, hydroxychloroquine is no longer being used to treat COVID-19 at Las Vegas-area hospitals including University Medical Center, which had prescribed the drug not only to gravely ill patients but to at-risk patients seen in the emergency room.
The hospital stopped its use after the Food and Drug Administration last week revoked emergency use authorization, citing “serious cardiac adverse events and other potential serious side effects.” The federal agency said hydroxychloroquine and its precursor, chloroquine, “showed no benefit for decreasing the likelihood of death or speeding recovery.”
playlist
In another blow, the National Institutes of Health on Saturday halted its study of hydroxychloroquine, saying that “while there was no harm, the study drug was very unlikely to be beneficial to hospitalized patients with COVID-19.”
Hydroxychloroquine is not the only drug that has “crashed and burned” as a possible treatment for COVID-19, said Dr. Shadaba Asad, medical director of infectious disease at the county hospital.
“We’ve looked at a lot of different modalities of treatment,” she said. “When we found that they’re not holding up to their initial promise, we stopped using them.
“The only reason this one got so much publicity was because of the politics that got involved.”
Politics and medicine
Hydroxychloroquine is approved by the FDA to prevent and treat malaria and also to treat autoimmune diseases such as rheumatoid arthritis and lupus.
President Donald Trump began touting hydroxychloroquine as a “game changer” for COVID-19 in March, the same month that the FDA granted its emergency use authorization, which allowed distribution of the federal government’s stockpile of the drug to hospitals across the country. In May, the president said he’d taken the drug to prevent COVID-19.
Doctors typically can prescribe drugs for purposes other than those approved by the FDA. However, interest in the drug became so intense that to prevent hoarding and shortages, Gov. Steve Sisolak on March 24 signed an emergency order limiting the drug’s use for COVID-19 to treating hospitalized patients, a move challenged by Nevada osteopaths. In early April, the state issued a dispensing waiver allowing hospitals to provide the drug to COVID-19 patients well enough to be sent home rather than admitted.
In mid-April, University Medical Center became the first hospital in Nevada to prescribe the drug on an outpatient basis to people seen in its emergency department who tested positive for COVID-19. Across the country, the drug was primarily used to treat COVID-19 in patients who were hospitalized or participating in clinical trials.
At the time, Dr. Thomas Zyniewicz, who heads the hospital’s emergency department, said that hydroxychloroquine was one of the most promising treatments available, giving high-risk patients “a fighting chance to not be on a ventilator.” Zyniewicz was unavailable for comment this week.
As with many things associated with hydroxychloroquine, the data has been controversial, with medical journal The Lancet retracting as unfounded a paper on a large study that concluded the drug could harm patients.
Asad said that the hospital did not prescribe the drug frequently enough on an outpatient basis to provide even anecdotal evidence of whether it had benefited patients. As with hospitalized patients given the drug, some improved while others did not, she said. When a patient did improve, it was unclear if they did so because of the drug or because they had a milder form of the disease.
The Valley Health System’s six hospitals, the Sunrise Health System’s four and St. Rose Dominican Hospitals’ three also are no longer using hydroxychloroquine to treat COVID-19 patients, representatives said.
Promising antiviral drug
In the race to find effective treatments for COVID-19, other drugs have gained prominence.
Most promising now, Asad said, is the antiviral drug remdesivir, which new research indicates stops the coronavirus from replicating.
In late April, a large, randomized, controlled trial sponsored by the National institute of Allergy and Infectious Diseases found that the drug sped up recovery from COVID-19 by four days.
“This might not sound like a lot, but it’s huge in terms of clinical treatments,” Asad said. “No other drug has shown this kind of benefit in COVID-19.”
After remdesivir received emergency use authorization from the FDA on May 1, it was in short supply across the country. Manufacturer Gilead Sciences donated supplies of the drug to the federal government, which distributed them to the states. Nevada received its first shipments of the drug in mid-May, according to the state Department of Health and Human Services. The state Board of Pharmacy allocates supplies to the Nevada hospitals based on their numbers of intensive care unit beds.
“We have limited supplies of remdesivir,” said Gordon Absher, spokesman for the St. Rose Dominican Hospitals. “With supply currently controlled by the federal government and allotted by the state, remdesivir has an uncertain supply chain.”
New data on steroid
In the past week, researchers announced that the common steroid dexamethasone may be beneficial in treating patients with COVID-19, in what Asad described as a significant turnabout.
“We had been told to stay away from steroids in (treating) COVID-19, and then all of a sudden this data comes out,” she said. Research by the University of Oxford in England suggested that the steroid may help severely ill patients by tamping down a heightened inflammatory response by the immune system.
“This is 180 degrees, right? Which is OK. We don’t get all excited,” said Asad, adding that details of the study have yet to be published. “We maybe try it, but all the same we watch our patients carefully. We look for adverse effects, and we see how things go. The thing is, politicians don’t get involved.”
With a chuckle, she added, “If tomorrow some politician comes and picks this up, it will become a mess.”
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.