Pill for treating COVID at home comes to Nevada, but in short supply
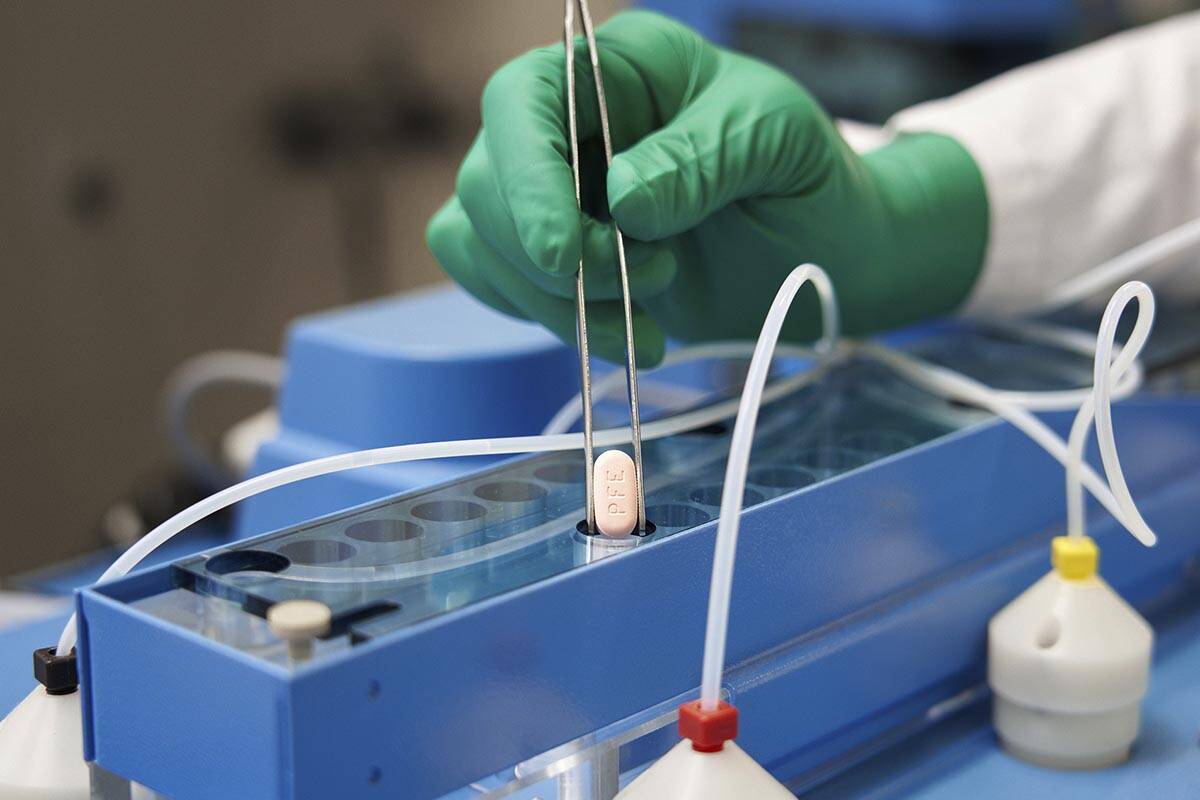
The first pill authorized in the U.S. for treating COVID-19 at home will initially be offered in Nevada primarily to patients in long-term care and skilled nursing facilities because of scarce supplies.
The remainder, less than 10 percent of the state’s initial allotment of Pfizer’s antiviral medication Paxlovid, has been given to University Medical Center in Las Vegas and to Renown Regional Medical Center in Reno, members of the Nevada State Board of Pharmacy said at a news briefing Thursday.
The drug’s authorization is “hands down, next to the vaccine, the most significant milestone in the pandemic,” said Dr. Shadaba Asad, the medical director of infectious disease at UMC.
Clinical trials indicate that the drug reduces the risk of hospitalization and death in high-risk individuals with COVID-19 by nearly 90 percent, results that Asad described as “mind-blowing.”
The U.S. Food and Drug Administration on Dec. 22 authorized the Pfizer pill for emergency use in those 12 and older with mild to moderate COVID-19 and at high risk for developing severe illness.
On Dec. 23, the FDA authorized a second pill to treat COVID-19 at home, molnupiravir, a medication developed by Merck and Ridgeback Biotherapeutics. Paxlovid is expected to become the top choice between the two drugs because studies indicate it has superior benefits and milder side effects.
Molnupiravir in clinical trials was shown to reduce the risk of hospitalization and death by 30 percent. The FDA said the drug should be considered for patients “for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate.” It also will carry a warning against use during pregnancy.
Molnupiravir will be allocated to retail pharmacies when it becomes available, state representatives said.
Limited supplies
Supplies of the Pfizer pill currently are extremely limited across the country. Nevada’s initial supplies are enough to treat 480 patients, with allocations expected to grow as production ramps up, pharmacy board executive secretary David Wuest said at Thursday’s COVID-19 briefing by state officials.
UMC has received a supply sufficient to treat 40 patients, which will be prioritized for those in the emergency department at greatest risk for developing severe COVID-19, Asad said in a phone interview. Some transplant patients, who are taking medication that suppresses their immune systems, may be good candidates for the pill. Other factors that heighten risk and would be considered include being unvaccinated, over the age of 65, and having conditions such as heart disease or diabetes, she said.
Pfizer’s pill is part of a decades-old family of antiviral drugs known as protease inhibitors, which revolutionized the treatment of HIV and hepatitis C. The drugs block a key enzyme that viruses need to multiply in the human body.
The FDA said the drug should be given within five days of the onset of symptoms. Treatment with the drug cocktail of two different medications consists of taking 30 tablets over five days. The agency does not not recommend Paxlovid for patients with severe kidney or liver impairment.
Paxlovid also can interact with heart medications and other commonly prescribed medications. The drug can slow down the metabolism of certain medications, and as a result “you end up with extremely high levels of those drugs,” Asad said.
“Now this, to me, is not an absolute contraindication to using this medication, but it does mean that it needs to be prescribed by someone who understands how this drug can interact with various medications,” she said.
Biggest impact may be in ER
Dr. David Weismiller, a family practitioner and professor at the Kirk Kerkorian School of Medicine at UNLV, described the new drugs as an “important step forward” in the treatment of COVID-19.
“For me in day-to-day practice, it’s probably not going to have a significant impact,” he said. He said he believes the drug will have the greatest impact in emergency departments that “are seeing those who are the sickest already and at risk for being hospitalized.”
He also said there would be shortages of the drug for the near future because of the complexity of creating the antiviral medication.
“For the general population, the way forward is still to make sure that you are immunized,” Weismiller said in an interview.
Asad envisions a day when Paxlovid, or another drug like it, becomes widely available to a broader swath of the population, much as antivirals are now prescribed for flu sufferers.
“So that the moment you’re diagnosed with COVID, and you even have mild symptoms, your physician can immediately prescribe an antiviral that is effective and you start that medication,” Asad said.
This approach, coupled with a population that is fully vaccinated and boosted, is the point at which “surely we’ll be able to turn this pandemic around,” she said.
The U.S. government has agreed to purchase enough Paxlovid to treat 10 million people, and it will be provided free to patients. Pfizer says it’s on track to produce 80 million courses globally next year, under contracts with the U.K., Australia and other nations.
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter. The Associated Press contributed to this report.