Feds and Las Vegas hospital partner to offer new COVID treatment
With its adult ICU full and its COVID-19 patient number at an all-time high, there was nonetheless new reason for hope Friday at Sunrise Hospital and Medical Center.
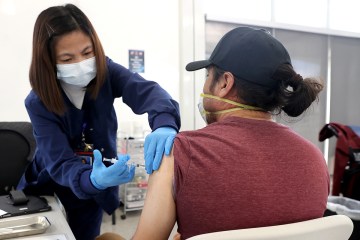
That hope came in the form of a big, white climate-controlled tent outside the medical center, one that in the afternoon began treating COVID-19 patients with a new therapy aimed at keeping them out of the hospital.
At a morning news conference, federal and hospital representatives announced that they had joined forces to open a temporary center to offer monoclonal antibody infusions, a promising new treatment for certain patients in the early stage of illness.
“It’s a wonderful event in the history of treatment of COVID-19 because previously, we’ve had so little to offer in this phase of illness,” said Dr. John Redd, chief medical officer for the assistant secretary for preparedness and response in the U.S. Department of Health and Human Services.
Monoclonal antibodies are molecules produced in laboratories that act as substitute antibodies, mimicking the immune system’s response to infection.
Monoclonal antibody treatments have been shown to decrease hospitalization rates in people at highest risk from severe disease from COVID-19, according to information from the federal health agency.
“These products can reduce the rate of hospitalization in people with risk factors from approximately 15 percent to 4 percent,” or by 70 percent, Redd said.
The center can treat about 32 patients a day. At an appointment, medicines are administered through an intravenous treatment called an infusion. The infusion takes about an hour, followed by an hour of observation. The one-time appointment takes about 2 ½ hours.
The center is only the third in the country to be opened with federal assistance. The other two are in Tucson, Arizona, and El Centro, California.
A federal medical team of 11 is partnering with Sunrise Hospital to set up the center and administer the treatments.
“The (federal) program called Operation Warp Speed has developed and then procured vaccines and therapeutics,” Redd said. “And in the case of vaccine and the new therapeutics, the cost to the person is zero. These products have been procured by the United States government on behalf of all Americans in pursuit of treatment of people all across the United States.”
Las Vegas was selected as the location for the center for several reasons.
“We do know that there is great need in Southern Nevada and around Las Vegas,” Redd said in an interview following the news conference. The federal government found “a very willing partner in Sunrise,” which also had the space and logistical ability to host the operation.
Outside of the three federally assisted centers, monoclonal antibody treatments are being administered in other medical settings across the country. However, health-care providers are administering just 20 percent of the doses they receive each week, according to officials with Operation Warp Speed, the Wall Street Journal reported.
This hesitancy was attributed to physicians’ lack of familiarity with the new treatment as well as patients’ limited interest in being treated at the onset of the disease, when it was unclear how ill they might become from the virus. Facilities also may not have the space and the staff to administer the treatments.
Even with promising early results, some doctors said they wanted to see more clinical data on the effectiveness of the treatment, which was authorized for emergency use by the Food and Drug Administration.
At the center at Sunrise, patients will receive the Eli Lilly drug bamlanivimab, authorized by the FDA on Nov. 9. It can be used to treat adult patients within 10 days of testing positive who are at high risk for progressing to severe COVID-19 or hospitalization due to certain risk factors. These risk factors include the following:
■ Body mass index of 35 or greater
■ Chronic kidney disease
■ Diabetes
■ Immunosuppressive disease
■ Receiving immunosuppressive treatment
■ 65 years of age or older
■ 55 years of age or older with cardiovascular disease, hypertension, COPD or another chronic respiratory disease
Patients who meet the criteria can be referred by their medical provider to receive the infusion treatment. Referring medical providers may call 702-961-9075 to schedule a patient.
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.