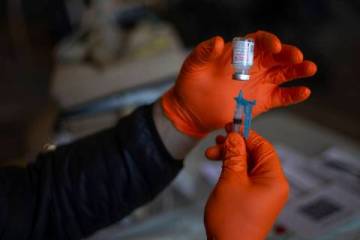
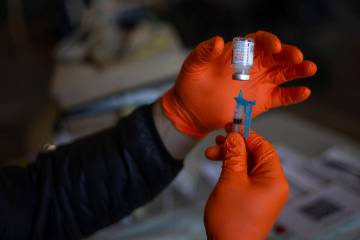
Las Vegas trial participants want to bring about COVID vaccine
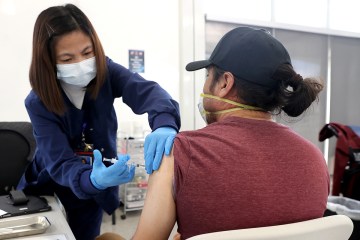
As of the past week, nearly half of the 490 Las Vegas-area participants in clinical trials for the Moderna COVID-19 vaccine had received their second injection of either the vaccine or a salt-water placebo.
Now, one or more participants just need to come down with the coronavirus.
“Those are the valuable jewels to find,” said Dr. Michael Levin, primary investigator for trials of the double-dose vaccine at Wake Research-Clinical Research Center of Nevada.
“So eventually we hope … it sounds terrible … somebody will get COVID,” said Levin, whose center is one of 98 around the country testing the Moderna pharmaceutical company’s vaccine on 30,000 volunteers. He quickly added that he wouldn’t wish the disease on anyone.
Yet a paradox of the trials is that some participants need to become ill with the very disease the vaccine is designed to thwart.
“The whole point of the trial, on the effectiveness side, is to measure whether the people with the vaccine have a lower rate of infection than the people who are taking the placebo,” said Dr. William Schaffner, professor of preventive medicine at the Vanderbilt University School of Medicine. “Then you can measure exactly what proportion of these infections were prevented.”
However, clinical trials traditionally attract individuals who are educated, health conscious and “very careful” when it comes to avoiding illness, he said.
“If so many of these volunteer are already very careful, the rate of infection will remain low, and it will take longer for these infections to accrue in the populations to make the assessment,” Schaffner said.
Levin agreed, saying that many participants are taking precautions against COVID-19 such as wearing masks and social distancing.
To determine the effectiveness of the vaccine, the Moderna trials across the country require that 150 participants become sick with the coronavirus, though researchers could make an interim assessment of effectiveness with as few as 50, Levin said. He did not know how many participants nationwide so far had contracted the virus.
The timing is random for when participants come down with COVID-19, a variable that contributes to uncertainty about when a vaccine might be ready. Some vaccine developers, including the World Health Organization, have been considering so-called “challenge studies” for the coronavirus, where volunteers agree to be intentially exposed to the virus, which promotes faster testing results.
There are four COVID-19 vaccines in late-stage clinical trials in the U.S., none of which involve challenge studies.
The federal government’s Operation Warp Speed program has set a goal of delivering the first doses of a safe and effective vaccine by January if not sooner. President Donald Trump has said a vaccine could be ready before the November election, though members of his administration have said a later date is more likely.
Hopeful participants
Las Vegans Ruth Sidorowicz, who is 91, and daughter Katie Craven fit the description of health-conscious clinical trial participants.
Both women, with Craven’s husband, are volunteers in the local Moderna study.
Because of the threat of the coronavirus, Sidorowicz doesn’t leave the house much except for an hourlong walk each morning with her daughter and cocker spaniel Gracie. She also exercises in the family’s pool each afternoon. And she is eager to return to twice-a-week bowling.
Sidorowicz met Katie’s father, a doctor, at the hospital in Rochester, N.Y, where she was training to be a nurse. She didn’t complete her training — the hospital told her she had to choose between nursing or getting married — but she has always been health conscious and interested in medical issues.
She chose to participate in the trial because she believes in the importance of vaccine studies and also “figured maybe they wouldn’t have that many people who are in their 90s, as I am,” she said.
The trial required that at least a quarter of participants be at higher risk for COVID-19 by virtue of being 65 or older or having underlying health conditions, according to Levin.
Craven, who is in her mid-60s and a pharmacist at Sunrise Hospital and Medical Center, takes precautions against the virus by wearing a mask and social distancing. Through her work as a pharmacist, she had assisted in conducting clinical trials in the past and was eager to participate as a volunteer in this trial.
“I like to travel, and this hopefully will be a way of opening up the rest of the world again,” she said about the promise of a vaccine. “COVID will hopefully be a thing of the past.”
Vaccine side effects
The family also wanted to participate in the trials to get early access to what could be a safe and effective vaccine. Those trial participants who receive the placebo are later offered the vaccine.
Trial participants aren’t told whether they are receiving vaccine or placebo. But Craven figures she got the vaccine because after her second injection, she experienced possible vaccine side effects of chills, fever and fatigue. She felt fine about a day later.
Sidorowicz she didn’t experience any symptoms after receiving her injections other than a bruise on her arm after the first injection.
Trial participants record any ill effects in an app. They also are in regular contact by phone with the research center between appointments.
Another vaccine trial participant, nurse Dawn Taylor, said that after receiving her first shot, she experienced a fever with redness, swelling and soreness at the injection site. She also was unusually tired for about two weeks.
Like the other trial participants interviewed for this article, she is limiting her in-person interactions with other people to avoid contracting the virus.
Taylor, 50, a lecturer in the nursing program at Nevada State College in Henderson, said she has joined at least five prior clinical trials, including for vaccines for yellow fever, dengue fever, H1N1 flu, bubonic plague and pneumonia. She compared the symptoms she experienced to those she had during the pneumonia vaccine trial. The only symptom this time that she considered unusual was that after the redness and swelling in her arm went away, it returned a week later before disappearing for good.
As a student pursuing her doctoral degree, she is drawn to vaccine trials by her interest in the research process. The $1,900 she will receive for participating in the Moderna trial over two years also will help to offset a pay reduction stemming from a pandemic furlough.
Her primary motivation, however, is her belief in the ethic of “pay it forward,” to do as previous generations had done to help develop vaccines for measles, mumps and many other maladies.
Vaccine trials, she said, require “healthy individuals to come forward and participate, to stand up and be counted today for future generations.”
Contact Mary Hynes at mhynes@reviewjournal.com or 702-383-0336. Follow @MaryHynes1 on Twitter.
How can I volunteer?
Those interested in participating in a Wake Research-Clinical Research Center study related to COVID-19 can text "COVID" to 702-357-9650 or visit covidstudies.org. To get a cross-section of the community, researchers are especially encouraging members of minority groups, as well as essential workers, to apply.