Nevada suspends use of Johnson & Johnson vaccine
Public health authorities in Nevada have called a temporary halt to the use of Johnson & Johnson’s COVID-19 vaccine, heeding the recommendation of federal regulators who are investigating six reports across the country of what may be a rare but serious side effect.
The Centers for Disease Control and Prevention and the Food and Drug Administration on Tuesday recommended a “pause” in using the single-dose vaccine to investigate reports of potentially dangerous blood clots, a development that could jeopardize the rollout of vaccines.
The agencies announced that they were investigating unusual clots that occurred six to 13 days after vaccination. The FDA commissioner said she expected the pause to last a matter of days.
Nevada quickly fell into line behind the new guidance.
“The State of Nevada is committed to protecting the health and safety of all Nevadans and will pause the use of the Janssen one-shot vaccine until the review is complete,” the state said in a news release, referring to the subsidiary that manufactures the J&J vaccine.
The clots occurred in veins that drain blood from the brain and occurred together with low platelets, the fragments in blood that normally form clots. All six cases were in women between the ages of 18 and 48. One person died, and all of the cases remain under investigation.
More than 6.8 million doses of the J&J vaccine have been given in the U.S., the vast majority with no or mild side effects.
Immediate impact
In Nevada as of April 12, more than 65,000 doses of the Janssen vaccine have been administered and recorded in Nevada WebIZ, according to Shannon Litz, a representative of the Nevada Department of Health and Human Services. More than 1.55 million doses of all vaccine types, including Pfizer-BioNTech and Moderna vaccines, have been administered, according to the state’s COVID-19 data portal.
The department said it is working with local partners to contact those scheduled to receive the J&J vaccine.
Like the state, the Southern Nevada Health district also said it was suspending administration of the vaccine and had advised its community partners to do the same.
To date, more than 1.1 million doses of vaccine have been administered in Clark County, including nearly 49,000 doses of the J&J vaccine, according to health district representative Jennifer Sizemore.
During a briefing by the health district, chief health officer Dr. Fermin Leguen said that only about 50 “minor adverse events” from the J&J vaccine, such as pain at the injection site, had been reported to date in the county.
Leguen did not expect the suspension of the vaccine’s use to have a major impact on vaccine availability locally, because the J&J vaccine has been a relatively small percentage of the supply here.
He noted, however, that the vaccine, which requires only one dose and is easier to store and handle, has been a useful tool for inoculating hard-to-reach populations.
When asked if Tuesday’s development could increase hesitancy about getting vaccinated, Leguen acknowledged it was a concern.
“Potential disaster”
Arthur Caplan, a nationally recognized authority on vaccination, put the potential impact in starker terms.
“I think it’s really a potential disaster for battling COVID,” Caplan, a medical ethicist at the NYU School of Medicine, told the Review-Journal. “This will fuel a lot of vaccine hesitancy, skepticism and doubt.”
He believes that the public may not differentiate that regulators’ concerns are about one particular COVID-19 vaccine, and not the Moderna or Pfizer vaccines. He noted that even if the blood clots are conclusively linked to the J&J vaccine, evidence suggests that the risk is remote.
“My message is take the vaccine,” said Caplan. “Don’t worry about the potential tiny, tiny risks.”
He said that it could make sense for some people, such as younger women, to choose a different type of vaccine if they have that option.
“But let’s not be paralyzed by a risk that is almost difficult to measure against the certainty that there are many people dying today of the disease,” he said.
Side effects taken seriously
Another top vaccine authority, Dr. Paul Offit, also hopes for a measured response from the public.
“People should be reassured that even very, very rare side effects are being taken seriously” by regulators, Offit said in a phone interview. Offit, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, served as a member of an independent committee that advised the FDA on COVID-19 vaccines.
He believes that the blot clots that have been identified are related to the vaccine’s technology, which uses a virus known as adenovirus as its platform. This is the same platform used by the vaccine developed by AstraZeneca and Oxford University, which also has been linked to rare blood clots in countries where it is being given. AstraZeneca has not sought approval for its vaccine in the U.S.
The FDA said the cases under investigation appear similar to the clots that are possibly linked to the AstraZeneca vaccine.
“I think this type of vaccine causes this type of very rare but serious side effect,” Offit said. “I think it was prudent for the FDA and CDC to take a breath.”
The Moderna and Pfizer vaccines use a different technology known as mRNA to carry instructions into the human body for making proteins that prime it to attack a specific virus, in this case the new coronavirus.
The CDC’s Advisory Committee on Immunization Practices (ACIP) is scheduled to meet Wednesday to review the six cases and make a recommendation.
One possible course of action would be to recommend that the vaccine be restricted to those over 55, for example, since the blood clots have been linked to younger women, Offit said. But he urged that the risk be put in perspective.
‘A matter of relative risks’
“It’s always a matter of relative risks. Here you have a virus that has killed 570,000 people,” he said. “There are advantages to having to give just one dose and some people may say, ‘You know, it’s one in a million chance of getting this.’”
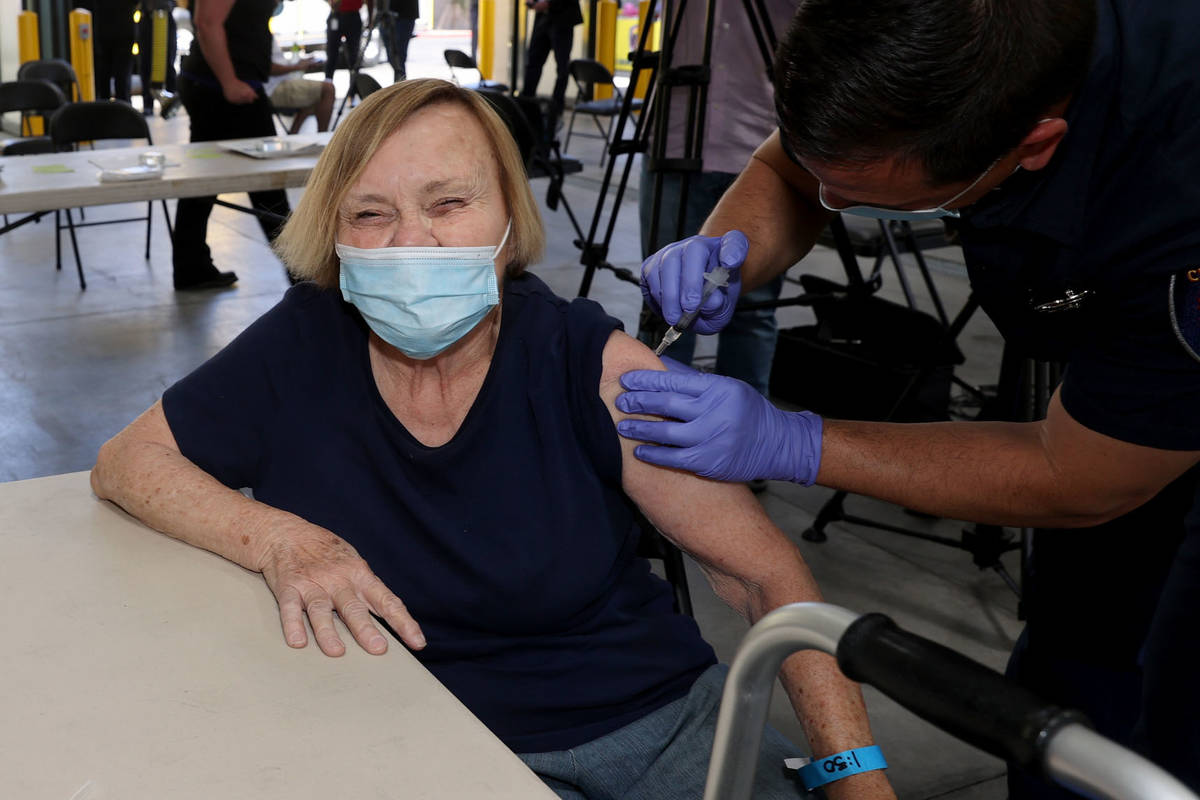
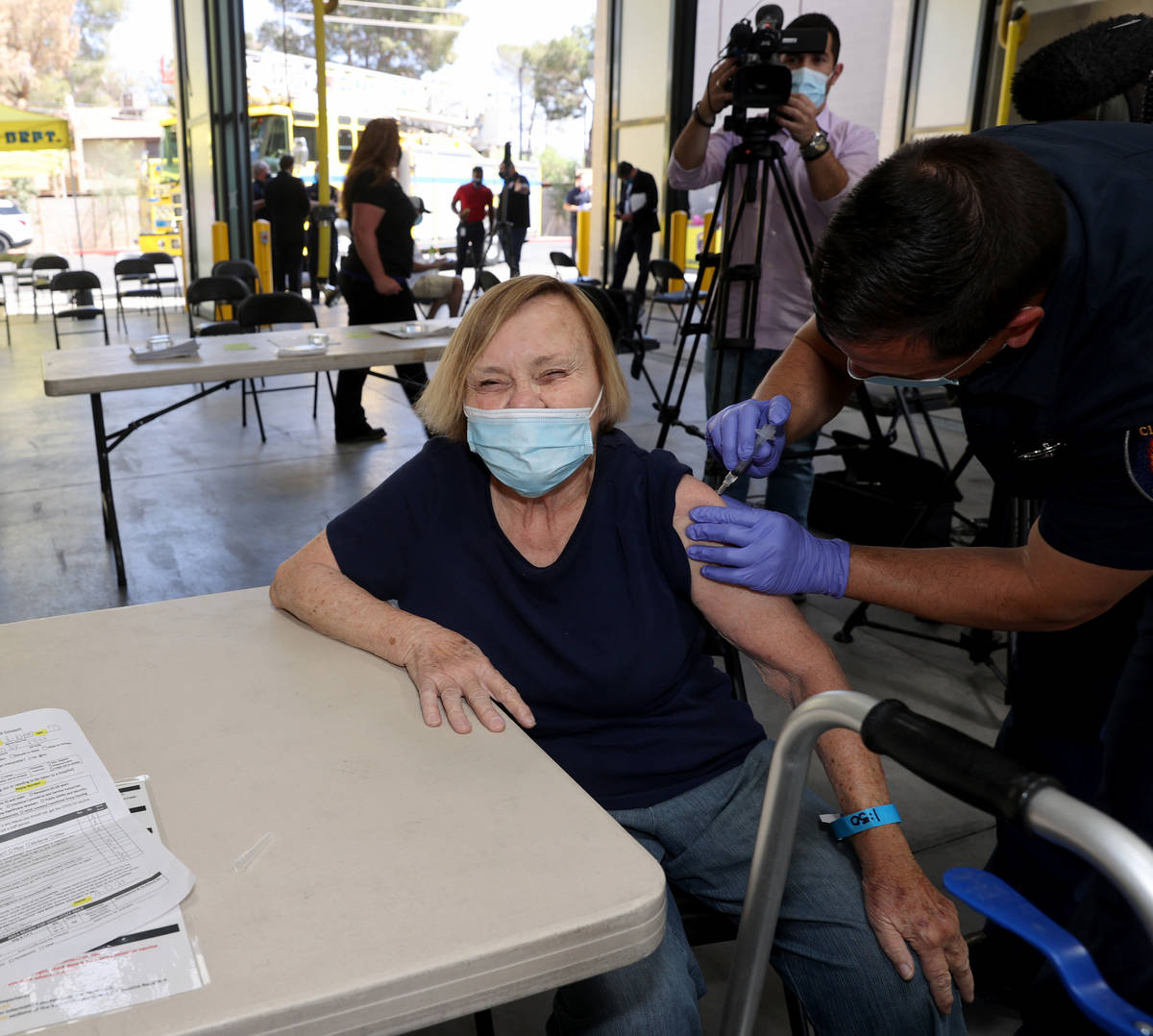
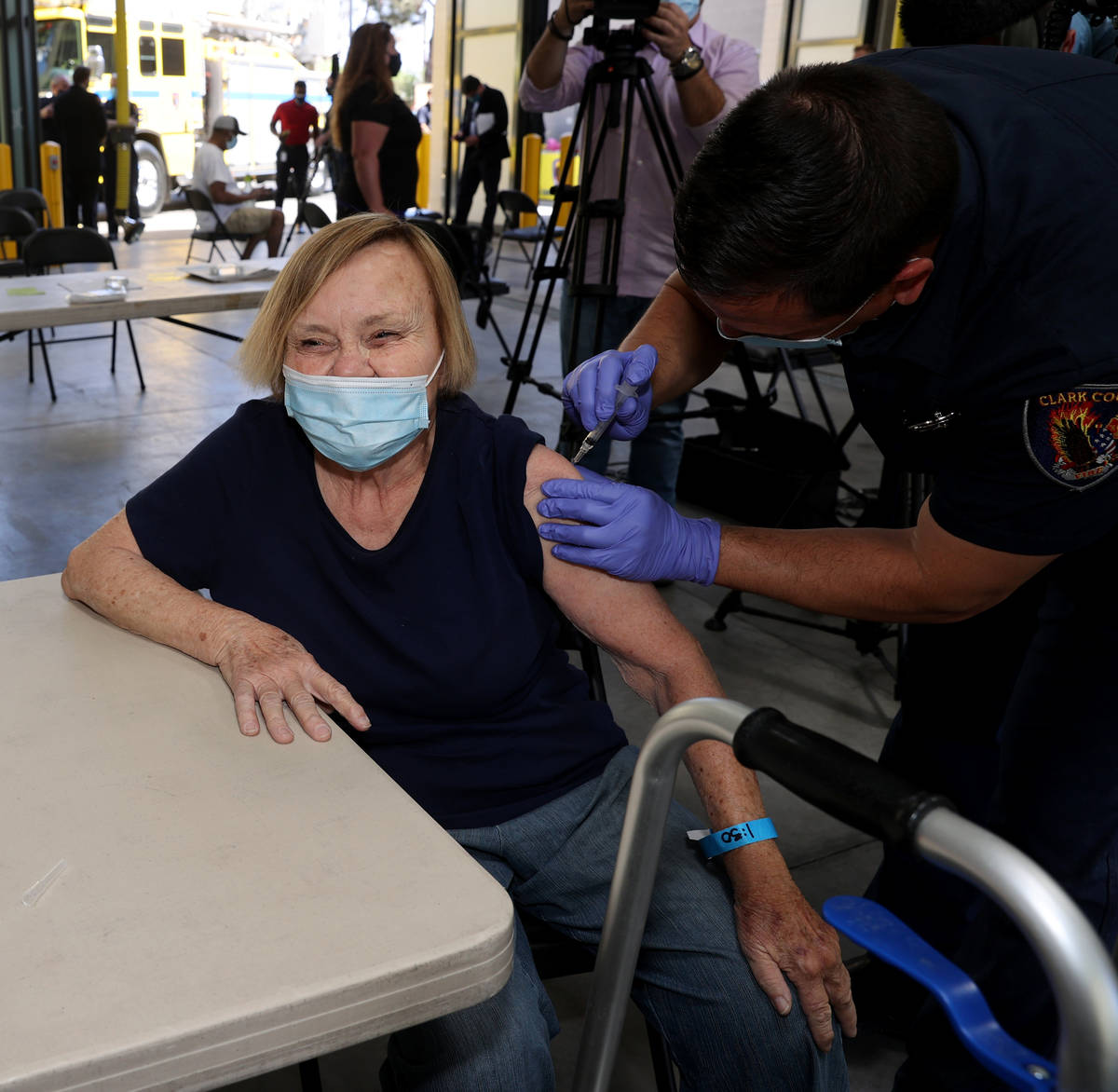
One Las Vegas resident who got the vaccine on Monday was untroubled by Tuesday’s development.
Darlene Blose, 77, of Las Vegas, said she drove more than 30 miles on Monday to be the first in line at an east Las Vegas fire station that was distributing J&J vaccines.
“I just feel more secure with one shot,” said Blose, adding that because she doesn’t have a computer, it would have been harder for her to schedule two appointments.
Blose heard Tuesday morning about the halt on the vaccine but was unconcerned.
Blose said even with the possibility of complications, she still would have gotten the vaccine. Now, she’s looking forward to having lunch with her friends for the first time in a year.
“We have to get through with it and try to get back to a normal life,” she said.
Contact Mary Hynes at mhynes@reviewjournal.com. Follow @MaryHynes1 on Twitter.
Review-Journal staff writers Katelyn Newberg and Michael Scott Davidson and the Associated Press contributed to this report.